Motivated and inspired by the roles of transition metals in biological settings, research work in the Tomat lab spans from fundamental studies of metal complexes and their electronic structures to medicinal applications of coordination chemistry. Our current projects aim at (i) the development of new approaches to target the role of iron in cancer growth, and (ii) the engineering of redox reactivity in metal complexes inspired by naturally occurring pigments.

Molecular strategies to examine and exploit the role of iron in cancer growth
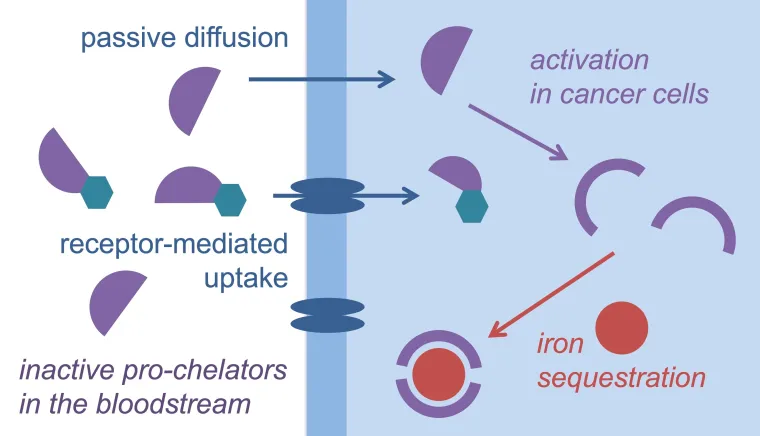
Malignant cells are characterized by increased iron acquisition and retention, which enable rapid proliferation rates. Among the physiological traits that differentiate cancer cells from normal cells, the higher demand for iron is emerging as a critical area of investigation with therapeutic potential. Although iron-sequestering compounds (i.e., chelators) have been successfully employed to treat iron overload for several decades, these pharmaceuticals primarily target extracellular labile iron in blood plasma. In contrast, the Tomat group works on the design and synthesis of prochelator systems that are activated for iron sequestration upon cellular entry. By introducing a disulfide bond in tridentate binding units of high-affinity iron scavengers (e.g., thiosemicarbazones, aroyl hydrazones), we have developed chelation systems that are activated for iron coordination upon reduction in the intracellular environment. In addition to acting as a reduction/activation switch, the disulfide linkage can be employed to form bioconjugates, for instance with carbohydrates or serum albumin. Furthermore, our work indicates that iron-binding strategies impact the tumor microenvironment through their effects on tumor-associated macrophages. This research program is funded by the National Institutes of Health (previously R01 GM127646 and R01 CA226920, currently R35 GM153364).
Development of redox-active ligands and luminescent radicals on the oligopyrrolic scaffolds of heme metabolites
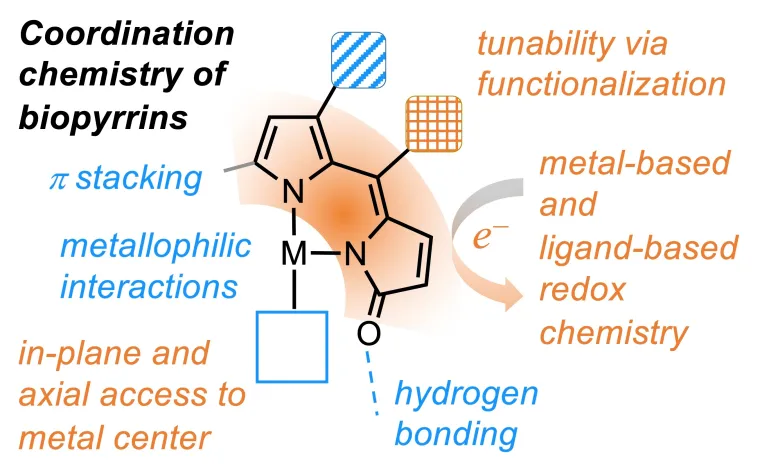
The stabilization of unpaired electrons within redox-active ligands adds to the available reactivity pathways and spin states of metal complexes for applications in catalysts, molecular magnets, and electronic devices. In the Tomat laboratory, naturally occurring pigments featuring the pyrrole heterocycle are the source of inspiration for our search of redox-active platforms for metal coordination. For instance, the ability of tetrapyrrolic macrocycles, such as porphyrin in heme, to host unpaired spins is critical to the catalytic activity of many enzymes (e.g., cytochrome P450 monooxygenase). With the goal of building more compact and versatile platforms for redox reactivity, we chose to investigate tripyrrolic and dipyrrolic fragments that were first isolated as products of heme metabolism in mammals. Our work is revealing that tripyrrindione and dipyrrindione pigments behave as stable redox-active ligands in transition metal complexes and present, in some cases, redox-responsive fluorescence emission. This research is funded by the National Science Foundation (previously CAREER 1454047 and currently CHE 2203361).
Experimental techniques
Our experimental approaches are rooted in synthetic chemistry and employ crystallographic and electrochemical methods along with a suite of spectroscopic techniques (e.g., optical absorption, fluorimetry, NMR, EPR, IR) for the characterization of ligands and metal complexes. In addition, we make use of molecular and cell biology methodologies to study the chemistry of our compounds directly in cultured mammalian cells.

